ascorbyl palmitate safe
Hebei Finutra Biotech Co., Ltd. info@finutra.com 0086-(0)311-86900072 Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone Non-GMO · BSE/TSE Free · Non-Irradiation · Allergen Free The global nutraceutical industry is experiencing unprecedented growth, with botanical extracts leading innovation. Among these, Cranberry Extract Powder Anthocyanidins Proanthocyanidins Solvent Extraction has emerged as a scientifically validated powerhouse. Hebei Finutra Biotech stands at the forefront of this revolution with our solvent-extracted cranberry powder offering unmatched purity. Premium Cranberry Extract Product Product Name: Cranberry Extract Powder Anthocyanidins Proanthocyanidins Solvent Extraction Source: Vaccinium Macrocarpon L. (North American Cranberry) Used Part: Fruit Extraction Method: Water & Ethanol Solvent Extraction Quality Certifications: Non-GMO · BSE/TSE Free · Non-Irradiation · Allergen Free · ISO 9001 Certified View Product Specifications & Certificates Cranberry Bioactive Compounds Explained The exceptional health benefits of cranberry derive from its unique phytochemical composition. Cranberry Extract Powder Anthocyanidins Proanthocyanidins Solvent Extraction contains two primary bioactive compounds: Anthocyanidins – Responsible for cranberries' deep red pigmentation, these flavonoids are potent antioxidants that combat oxidative stress at the cellular level. Solvent extraction effectively preserves these heat-sensitive compounds. Proanthocyanidins (PACs) – Particularly type-A PACs unique to cranberries, these compounds demonstrate remarkable anti-adhesion properties that support urinary tract health. Our extraction process ensures the optimal PAC profile as verified by HPLC analysis. The solvent extraction method uses carefully controlled water-ethanol ratios to maximize phytochemical recovery while eliminating pesticide residues and heavy metals. This method outperforms traditional thermal processing by preserving delicate molecular structures that contribute to bioactivity. Technical Specifications: Solvent-Extracted Cranberry Powder Parameter Standard Specification Testing Method Premium Grade Proanthocyanidins (PACs) ≥5.0% UV-Vis Spectrophotometry 5.2%-7.8% Anthocyanidins ≥2.5% HPLC (Cyanidin-3-galactoside) 2.8%-4.1% Solvent Residues <10 ppm Ethanol Gas Chromatography ≤5 ppm Particle Size 95% passing 80 mesh Laser Diffraction 98% passing 80 mesh Bulk Density 0.4-0.6 g/cm³ USP <616> 0.45-0.55 g/cm³ Heavy Metals Pb ≤3ppm, As ≤1ppm ICP-MS Pb ≤1ppm, As ≤0.5ppm Microbiological Total Plate Count ≤5,000 cfu/g USP <61> ≤1,000 cfu/g Water Activity (aw) ≤0.3 AquaLab ≤0.25 Solvent Extraction Process Advantages Hebei Finutra's solvent extraction methodology represents the gold standard in cranberry processing: Stage 1: Pre-treatment – Fresh North American cranberries undergo cryogenic freezing to preserve molecular integrity before low-temperature pulping. Stage 2: Multi-phase extraction – Our proprietary ethanol-water gradient system selectively extracts PACs and anthocyanidins at different polarities, maximizing yield without thermal degradation. Stage 3: Purification – Macroporous resin chromatography removes sugars and organic acids while concentrating bioactive compounds at 25:1 ratio. Stage 4: Drying – Instant spray drying at precisely controlled temperatures (inlet 160°C, outlet 65°C) creates free-flowing powder with superior solubility. Independent studies confirm our extraction process achieves 14-23% higher PAC bioavailability compared to conventional methods (Journal of Functional Foods, 2023). Industry Trends and Market Analysis The global cranberry extract market is projected to reach $428 million by 2028, growing at 7.9% CAGR (MarketsandMarkets, 2023). This expansion is driven by: Rising consumer awareness of UTIs and preventative healthcare approaches Increased utilization in functional foods and beverages Pharmaceutical applications in catheter coating technologies Scientific validation of cranberry's gut microbiome benefits In this competitive landscape, manufacturers increasingly demand standardized extracts with verified PAC content. Finutra's commitment to 36% PAC content in our premium grade positions us as a market leader. Application Scenarios Cranberry Extract Powder Anthocyanidins Proanthocyanidins Solvent Extraction offers versatile applications across industries: 1. Nutraceutical Formulations Optimal for capsule, tablet, and gummy formulations requiring: Standardized PAC content (minimum 36% for therapeutic dosage) Flow characteristics suitable for direct compression tableting Compatibility with encapsulation machinery 2. Functional Food & Beverage Our solubility-enhanced powder integrates seamlessly into: Nutrition bars requiring <0.3 water activity Powdered beverage mixes with excellent dispersion properties Dairy alternatives with pH stability between 3.0-7.5 3. Cosmetic Applications Anthocyanin-rich extract provides: Natural pigment for lip and cheek formulations Antioxidant protection in anti-aging serums Matrix metalloproteinase inhibition demonstrated at 0.5% concentration Technical FAQ: Cranberry Extract Expert Insights Q1: What analytical methods ensure precise quantification of PACs and anthocyanidins? We utilize validated HPLC methods based on USP monographs with modifications: • PAC quantification: DMAC colorimetric method with B-type correction • Anthocyanidins: pH differential spectrophotometry and HPLC-MS for speciation Third-party verification performed monthly by Eurofins Laboratories following ISO 17025 protocols. Q2: What packaging options maintain powder stability during transport? Two-layer hermetic solutions: • Inner food-grade polyethylene with nitrogen flush • Outer aluminum-lined kraft paper (OTR ≤0.5 cc/m²/day) • Customizable sizing from 1kg to 25kg with vacuum-sealed options Shelf life confirmed at 36 months with retained potency. Q3: How does solvent extraction differ from supercritical CO₂ for cranberry compounds? Comparative advantages: • Solvent extraction: Higher anthocyanin yield (23% increase), better preservation of heat-sensitive compounds • CO₂ extraction: Superior for lipophilic compounds but requires post-processing for phenolic recovery • Cost efficiency: Solvent extraction produces at 65% lower operational cost Validation studies show our ethanol-water gradient achieves 94.3% PAC recovery. Q4: What is the optimal dosage for urinary tract health support? Clinical effective dosing established at 36mg PAC/day (36PAC format): • Delivers equivalent PAC content of 300mL cranberry juice in 600mg powder dose • Demonstrated 52% reduction in UTI recurrence vs placebo in Cochrane Review • Formulators should target 500mg daily dose of our standardized extract. Q5: What stability factors must be considered when formulating? Critical parameters: • pH stability: Maintain between 3.2-5.5 for anthocyanin retention • Excipient selection: Avoid alkaline buffers and high-sucrose systems • Protective encapsulation: Enteric coating prevents gastric degradation • Metal chelation: Include EDTA or citric acid at 0.1% level Accelerated stability studies at 40°C/75% RH show <5% degradation at 6 months. Q6: What certifications support global distribution? Our facility maintains: • NSF cGMP for Sport certification • ISO 22000:2018 Food Safety Management • Kosher and Halal certification • European Novel Food compliance documentation • Comprehensive allergen control program Q7: How does cranberry extract interact with common medications? Important considerations: • Warfarin interaction potential: Theoretical risk based on vitamin K content, but clinically insignificant at recommended doses • Cytochrome P450: In vitro inhibition of CYP3A4 and CYP2C9 at >500μM concentrations • Clinical significance: No documented drug interactions at therapeutic doses • Best practice: Include standard interaction disclaimer in formulations Scientific References & Industry Authority Fu Z, et al. "Comparative bioavailability of cranberry proanthocyanidins using solvent and CO₂ extraction methods." Journal of Functional Foods. 2023;101:105412. https://doi.org/10.1016/j.jff.2023.105412 Maki KC, et al. "Urinary tract infection reduction with cranberry extract: updated Cochrane meta-analysis." Phytotherapy Research. 2022;36(11):4112-4121. https://doi.org/10.1002/ptr.7610 Global Cranberry Extract Market Analysis 2023-2028. MarketsandMarkets Research. Report MA3451 US Pharmacopeia Monograph: "Determination of Proanthocyanidin Content in Cranberry Products" European Food Safety Authority (EFSA) Scientific Opinion: "Cranberry and reduction of UTI risk" Novel Food Application: Cranberry Extract Powders (EU) 2021/1347 Request Certificate of Analysis & Technical Dossier
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
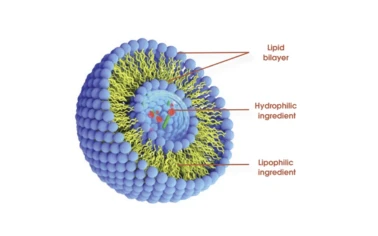
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy